Shuang Wu
Professor of Plant Development
1000 Young Talents Program in China, NSF Outstanding Youth
Contact Information
Haixia Institute of Science and Technology (HIST)
Fujian Agriculture and Forestry University (FAFU)
15 Shang xia dian Road
Fuzhou, Fujian 350002, P. R. China
Phone: 86-591-86393541
Email: wus@fafu.edu.cn
Lab Webpage:
https://wusplant.wixsite.com/shuang-wu
Education
2003-2009, Ph.D., University of Massachusetts, US
2000-2003, M.S., University of Science & Technology of China, Hefei, China
1996-2000, B.S., Anhui Agricultural University, Hefei, China
Professional Experience
2015-Present, Professor, Fujian Agriculture and Forest University. China
2009-2014, Postdoctoral Fellow, University of Pennsylvania
Research Interests
As sessile organisms, plants acquire plasticity in adverse environments partially by regulating their postembryonic growth, which largely relies on two processes: oriented cell division and cell fate determination. A major goal of my research is to understand how cell division and cell fate determination of the plant root system are spatiotemporally controlled. A second direction in my lab is to study how the stem cell regulation participates in plant organogenesis.
- Pattern formation during organogenesis in plants
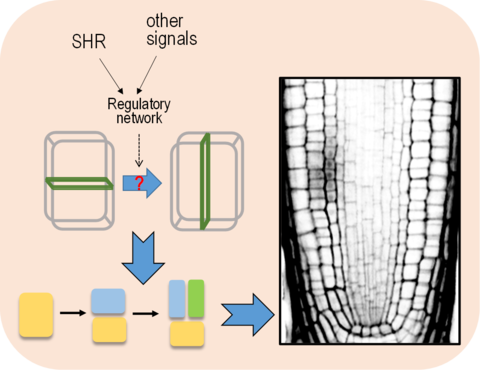
An enduring question in developmental biology is how cellular patterning and specification of cell fate are achieved. Pattern formation in plants is a process in which specialized cells are organized at defined times and in specific positions. In the plant root system, the patterning process starts from oriented cell division in stem cell niche followed by the specification of distinct cell fates. A good example is the ground tissue formation in the Arabidopsis root. To separate the ground tissue into endodermis and cortex, a critical regulator SHR that is synthesized in stele moves outwards into endodermal/cortex initial cells where SHR promotes asymmetric division and confers endodermal identity. However, the regulatory network mediated by SHR is only partially known. In particular, the cellular mechanisms that re-orient spindle and cell plate in response to SHR signaling is entirely unknown. Using the combination of genomics, genetics, cellar and molecular biology, we try to elucidate what cellular events and molecular regulators account for the rotation of division direction (shown in figure 1).
Figure. 1
- Stem cell regulation and cell fate specification
Plants have the ability to produce new organs continuously, which relies on the maintenance of stem cells in both shoot apical meristem and root apical meristem. In Arabidopsis, we focus on studying how stem cells are maintained in the stem cell niche (SCN) at the root tip. In tomato, we choose floral meristem as the working system as it directly relates to the tomato fruit formation.
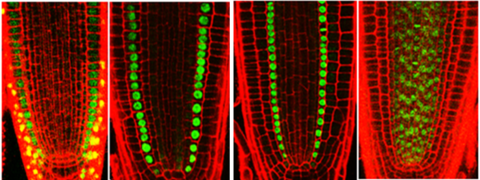
Unlike animal cells, plant cells are not able to change their position and orientation. Thus, position-dependent cell fate determination is the basic principle of plant development. We try to gain a comprehensive view of how plants translate various positional cues into transcriptional re-programmings that specify different cell fates (Figure 2).
Figure 2
- Trichome formation in tomato
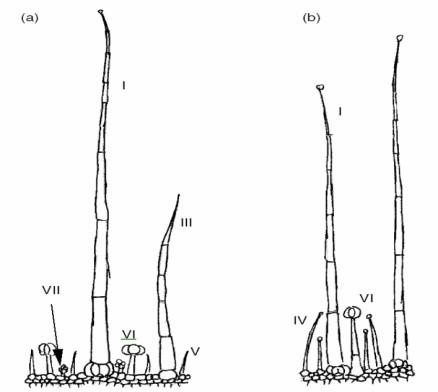
Trichomes are uni- or multi-cellular structures that are formed by the epidermis of most plants and act as a protective layer against biotic and abiotic stresses (Figure 3). In tomato, trichomes are multicellular structure and can be classified into seven different types based on cell number, shape and the presence of glandular cells. We are interested in the whole developmental processes by which seven different tomato trichomes are formed.
Figure 3. Tomato trichomes (Luckwill, 1943)
Publications
Hua B, Chang J, Han X, Xu Z, Xu M, Yang M, Yang C, Ye Z, Wu S.* (2020) HOMEODOMAIN PROTEIN8 Mediates Jasmonate-Triggered Trichome Elongation in Tomato. New Phytologist In press
Xu M, Gu X, Yu Q, Liu Y, Bian X, Wang R, Yang M, Wu S.* (2020) Time-course observation of the reconstruction of stem cell niche in the intact root. Plant Physiology In press
Yang L, Qi S, Touqeer A, Li H, Zhang X*, Liu X*, Wu S.* (2020) SlGT11 controls floral organ patterning and floral determinacy in tomato. BMC Plant Biology 20:562
Hua B, Chang J, Wu M, Xu Z, Zhang F, Yang M, Xu H, Wang L, Chen XY, Wu, S.* (2020) Mediation of JA signaling in glandular trichomes by the woolly/SlMYC1 regulatory module improves pest resistance in tomato. Plant Biotechnology Journal doi: 10.1111/pbi.13473.
Wu J, Cheng J, Xu C, Qi S, Sun W, Wu, S.* (2020) AUREA maintains the balance between chlorophyll synthesis and adventitious root formation in tomato. Horticulture Research 7:166. doi: 10.1038/s41438-020-00386-x.
Li P, Cai Q, Wang H, Li S, Cheng J, Li H, Yu Q, Wu, S.* (2020) Hydrogen peroxide homeostasis provides beneficial micro-environment for SHR-mediated periclinal division in Arabidopsis root. New Phytologist doi: 10.1111/nph.16824.
Wang C, Wang H, Li P, Li H, Xu C, Cohen H, Aharoni A, Wu, S.* (2020) Developmental programs interact with ABA to coordinate root suberization in Arabidopsis. Plant J. doi: 10.1111/tpj.14920.
Zhang L, Zhao Y, Liang H, Li X, Gallagher KL*, Wu, S.* (2020) Gateway-compatible vectors for functional analysis of proteins in cell type specific manner. Plant Methods doi:10.1186/s13007-020-00635-z.
Dong W, Zhu Y, Chang H, Wang C, Yang J, Shi J, Gao J, Yang W, Lan L, Wang Y, Zhang X, Dai H, Miao Y, Xu L, He Z, Song C, Wu S, Wang D, Yu N, Wang E. (2020) An SHR-SCR module specifies legume cortical cell fate to enable nodulation. Nature doi: 10.1038/s41586-020-3016-z.
Cohen H, Fedyuk V, Wang C, Wu S, Aharoni A. (2020) SUBERMAN regulates developmental suberization of the Arabidopsis root endodermis. Plant J. 102(3):431-447.
Wang Z, Rong D, Chen D, Xiao Y, Liu R, Wu S, Yamamuro C. (2020) Salicylic acid promotes quiescent center cell division through ROS accumulation and down-regulation of PLT1, PLT2, and WOX5. J Integr Plant Biol. doi: 10.1111/jipb.13020.
Chang J, Xu Z, Li M, Yang M, Qin H, Yang J, Wu, S.*(2019)Spatiotemporal cytoskeleton organizations determine morphogenesis of multicellular trichomes in tomato. PLoS Genetics 15(10): e1008438.
Xu M, Gu X, Liang N, Bian X, Wang H, Qin Y, Pi L, Wu, S.* (2019) Intersected functional zone of transcriptional regulators patterns stemness within stem cell niche of root apical meristem. J Integr Plant Biol. 62(7):897-911.
Spiegelman Z, Wu, S, Gallagher KL (2019) A role for the endoplasmic reticulum in the cell-to-cell movement of SHORT-ROOT. Protoplasma 256(5):1455-1459.
Wu ML, Cui YC, Ge L, Cui LP, Xu ZC, Zhang HY, Wang ZJ, Zhou D, Wu S, Chen L, Cui H. (2019) NbCycB2 represses Nbwo activity via a negative feedback loop in tobacco trichome development. J Exp Bot. 71(6):1815-1827.
Qi SL, Lin QF, Feng XJ, Han HL, Liu J, Zhang L, Wu S, Le J, Blumwald E, Hua XJ. (2019) IDD16 negatively regulates stomatal initiation via trans-repression of SPCH in Arabidopsis. Plant Biotechnol J. 17(7):1446-1457.
Li, P., Yu, Q., Gu, X., Xu, C., Qi, S., Wang, H., Zhong, F., Baskin, T.B., Rahman, A., Wu, S.* (2018) Construction of a Functional Casparian Strip in Non-endodermal Lineages Is Orchestrated by Two Parallel Signaling Systems in Arabidopsis thaliana. Current Biology 28(17):2777-2786.e2.
Xu, M., Qi, S., Chen, F., Zhang, L., Wu, S.* (2018) Loss or duplication of key regulatory genes coincides with environmental adaptation of stomatal complex in Nymphaea colorata and Kalanchoe laxiflora. Horticulture Research 5:42. doi: 10.1038/s41438-018-0048-8.
Li, P., Yang, M., Chang, J., Wu, J., Zhong, F., Rahman, A., Qin, H. and Wu, S.* (2018) Spatial Expression and Functional Analysis of Casparian Strip Regulatory Genes in Endodermis Reveals the Conserved Mechanism in Tomato. Front. Plant Sci. 9:832. doi: 10.3389/fpls.2018.00832
Zhong, F., Wang, S., Roan, S., Lin, B., Chen, I., Lin, Y., Pang, J., Wu, S.* (2018) Characterization of nitrate assimilation in Lactuca sativa L. under different nitrogen sources. Plant Growth Regulation https://doi.org/10.1007/s10725-018-0404-6.
Yu Q, Li P, Liang N, Wang H, Xu M, Wu, S. *(2017)Specifying cell fate in roots requires coordinative lineage instruction and positional reprogramming. Plant Physiol. 175(2):816-827.
Liu, Y., Xu, M., Liang, N., Zheng, Y., Yu, Q., Wu, S. * (2017) Symplastic communication spatially directs local auxin biosynthesis to maintain root stem cell niche in Arabidopsis, Proc Natl Acad Sci U S A. 114(15):4005-4010. (Faculty of 1000)
Yang, B., Zhou, X., Xu, R., Wang, J., Lin, Y., Pang, J., Wu, S. *, Zhong, F. * (2016) Comprehensive Analysis of Photosynthetic Characteristics and Quality Improvement of Purple Cabbage under Different Combinations of Monochromatic Light, Front Plant Sci. 29;7:1788. (*Co-corresponding author)
Wu, S.*, O'Lexy, R., Xu, M., Sang, Y., Chen, X., Yu, Q. & Gallagher, K.* (2016) A Symplastic signaling instructs cell division, cell expansion, and cell polarity in the ground tissue of Arabidopsis thaliana roots, Proc Natl Acad Sci U S A. 113(41):11621-11626. (*Co-corresponding author)
Wu, S., Hayashi, T., Lee, C.M., Price, S., Divol, F., Henry, S., Pauluzzi, G., Perin, C. & Gallagher, K. (2014) A plausible mechanism, based upon SHORT-ROOT movement, for regulating the number of cortex cell layers in roots, Proc Natl Acad Sci U S A. 111(45):16184-9.
Wu, S. & Gallagher, K.L. (2014) The movement of the non-cell-autonomous transcription factor, SHORT-ROOT relies on the endomembrane system, Plant J. 80(3):396-409. (Faculty of 1000)
Wu, S. & Gallagher, K.L. (2015) Techniques for Assessing the Effects of Pharmacological Inhibitors on Intercellular Protein Movement. Methods in Molecular Biology. 1217:245-58.
Wu, S. & Gallagher, K.L. (2013) Intact microtubules are required for the intercellular movement of the SHORT-ROOT transcription factor. Plant J. 74(1):148-159. (Faculty of 1000)
Wu, S., Baskin, T.I. & Gallagher, K.L. (2012) Mechanical fixation techniques for processing and orienting delicate samples, such as the root of Arabidopsis thaliana, for light or electron microscopy. Nature Protocols. 7(6):1113-24.
Wu, S. & Gallagher, K.L. (2012) Transcription Factors on the Move. Curr Opin Plant Biol. 15(6):645-651.
Koizumi, K.*, Wu, S.* (co-first author), MacRae-Crerar, A., Gallagher, K.L. (2011) An essential protein that interacts with endosomes and promotes movement of the SHORT-ROOT transcription factor. Current Biology. 21(18):1559-1564. (Faculty of 1000)
Wu, S. & Gallagher, K.L. (2011) Mobile protein signals in plant development. Curr Opin Plant Biol. 14(5):563-70
Wu, S.*, Koizumi, K., Macrae-Crerar, A., Gallagher, K.L. (2011) Assessing the utility of photoswitchable fluorescent proteins for tracking intercellular protein movement in the Arabidopsis root. PLoS One. 2011; 6(11):e27536. (Faculty of 1000) (Selected by the illuminated plant cell as paper of the month, Feb 2012)
Wu, S., Scheible, W. R., Schindelasch, D., Van Den Daele H., De Veylder L., & Baskin, T. I. (2010) A conditional mutation in Arabidopsis thaliana separase induces chromosome non-disjunction, aberrant morphogenesis and cyclin B1;1 stability. Development. 137(6):953-961. (Faculty of 1000)
Wu, S., Gu, Y., Zhao, L. & Liu, J. (2003) Effects of synchrotron radiation from soft X-rays on peroxidation products of membrane lipids and activity of related enzymes in wheat seeds. Nuclear Techniques. 26: 577-581.
Chen, X., Wu, S., Liu, Z., Friml, J. (2016) Environmental and Endogenous Control of Cortical Microtubule Orientation. Trends Cell Biol. 26(6):409-19.
Koizumi, K., Hayashi, T., Wu, S., Gallagher, K.L. (2012) The SHORT-ROOT protein acts as a mobile, dose-dependent signal in patterning the ground tissue. Proc Natl Acad Sci U S A. 109(32):13010-5. (Faculty of 1000)
Sang, Y., Silva-Ortega, C.O., Wu, S., Yamaguchi, N., Wu, M.F., Pfluger, J., Gillmor, S., Gallagher, K.L., Wagner, D. (2012) Mutations in two non-canonical Arabidopsis SWI2/SNF2 chromatin remodeling ATPases cause embryogenesis and stem cell maintenance defects. Plant J. 72(6): 1000-1014.
Vatén, A., Dettmer, J., Wu, S., Stierhof, Y.D., Miyashima, S., Yadav, S.R., Roberts, C.J., Campilho, A., Bulone, V., Lichtenberger, R., Lehesranta, S., Mähönen, A.P., Kim, J.Y., Jokitalo, E., Sauer, N., Scheres, B., Nakajima, K., Carlsbecker, A., Gallagher, K.L., Helariutta, Y. (2011) Callose biosynthesis regulates symplastic trafficking during root development. Developmental Cell. 21(6):1144-1155. (Faculty of 1000)
Yang, X., Boateng, K.A., Yuan, L., Wu, S., Baskin, T.I., Makaroff, C.A. (2011) The radially swollen 4 separase mutation of Arabidopsis thaliana blocks chromosome disjunction and disrupts the radial microtubule system in meiocytes. PLoS One. 29;6(4):e19459.
Rahman, A., Takahashi, M,, Shibasaki, K., Wu, S., Inaba T., Tsurumi, S. & Baskin, T. I. (2010) Gravitropism of Arabidopsis thaliana roots requires the polarization of PIN2 toward the root tip in meristematic cortical cells. Plant Cell. 22(6):1762-76
Bischoff, V., Cookson, S. J., Wu, S. & Scheible, W. R. (2009) Thaxtomin A affects CESA-complex density, expression of cell wall genes, cell wall composition, and causes ectopic lignification in Arabidopsis thaliana seedlings. J. Exp. Bot. 60(3): 955-65.